Anticholinergic Urinary Retention Risk Calculator
Risk Assessment Tool
This tool helps clinicians assess urinary retention risk in patients taking anticholinergic medications based on clinical parameters from current guidelines.
Risk Assessment Results
Key Risk Factors:
Clinical Guidance
When a patient takes anticholinergic medications are drugs that block acetylcholine at muscarinic receptors, reducing involuntary bladder muscle contractions, they may unintentionally interfere with the normal emptying of the bladder. This side effect-known as anticholinergic urinary retention-can range from a slight feeling of incomplete voiding to a full‑bladder emergency that requires catheterisation.
How Anticholinergic Drugs Work
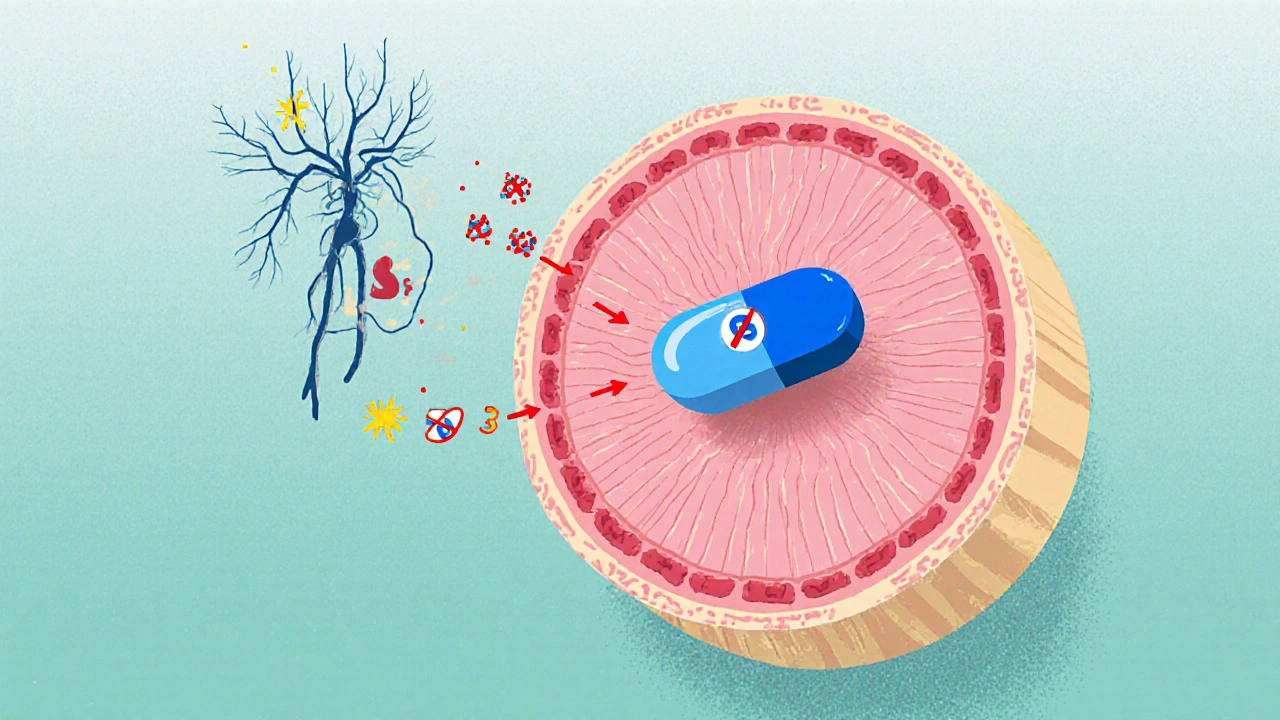
Anticholinergics bind to the five muscarinic receptor subtypes (M1‑M5). In the urinary tract, the detrusor muscle primarily relies on M3 receptors to contract and push urine out. By antagonising M3, these drugs increase functional bladder capacity and reduce urgency, which is why they are prescribed for overactive bladder (OAB). However, the same mechanism also dampens the contractile force needed for a complete void, especially when other factors already weaken bladder outflow.
Why Blocked Muscarinic Activity Leads to Retention
The micturition cycle is a coordinated dance of nerves and muscles:
- Parasympathetic nerves release acetylcholine, activating M3 receptors on the detrusor.
- Sympathetic input relaxes the internal sphincter via α1‑adrenergic receptors.
- Somatic nerves keep the external sphincter relaxed by reducing nicotinic activity.
If any step is disrupted-especially the detrusor contraction-urine lingers. Studies show that an anticholinergic burden score of 3 or higher on the Anticholinergic Cognitive Burden (ACB) scale raises the odds of retention by nearly 70 % in older adults (JAGS 2017).
Who Is Most at Risk?
Risk isn’t uniform. Certain groups face a higher chance of developing retention:
- Men over 65 with benign prostatic hyperplasia (BPH): prostate enlargement already narrows the outlet, so adding an anticholinergic can push the residual volume from a safe 80 mL to >150 mL, triggering acute retention.
- Patients on multiple anticholinergic‑scoring drugs (polypharmacy), especially when combined with opioids or antihistamines.
- Individuals with baseline post‑void residual (PVR) >100 mL before starting therapy.
- Elderly patients with cognitive impairment, because the same cholinergic blockade worsens both brain and bladder function.
Data from a 2019 University of Calgary review estimate a 4.3 % incidence of drug‑induced retention in men with BPH, compared with just 0.5 % in the general population.
Comparing Retention Risk Across Common Anticholinergics
Not all anticholinergics carry the same danger. Selectivity for the M3 receptor and the ability to cross the blood‑brain barrier influence how likely a drug is to cause urinary problems.
| Drug | Receptor selectivity | Typical retention incidence (%) | Special notes |
|---|---|---|---|
| Oxybutynin | Balanced M1‑M3 affinity | 1.8‑2.5 (high) | Oral form shows 42 % higher risk vs. patch |
| Tolterodine | Moderate M2‑M3 affinity | 1.2‑1.8 (moderate) | Extended‑release lowers peak levels |
| Solifenacin | 31‑fold M3 over M2 | 1.2‑1.8 (moderate) | Effective at low doses; monitor PVR |
| Darifenacin | Highly selective M3 | ~1.5 (moderate‑high) | Less cognitive impact, still needs bladder monitoring |
| Trospium chloride | High M1‑M3 affinity, limited CNS penetration | 1.5‑2.2 (moderate‑high) | Often chosen for patients with dementia risk |
| Mirabegron (β3‑agonist) | Stimulates β3 receptors - opposite mechanism | 0.3 (low) | Preferred in men with BPH |
Overall, non‑selective agents like oxybutynin carry roughly three times the odds of retention compared with placebo in men with BPH (NEJM 2009). The hierarchy above aligns with the 2018 European Association of Urology guideline.
Monitoring and Prevention Strategies
Guidelines from the American Urological Association (2022) require a baseline PVR measurement before starting any anticholinergic in men. The recommended workflow looks like this:
- Perform bladder ultrasound; record PVR.
- If PVR ≤150 mL, start the lowest possible dose (often 25 % of the adult dose).
- Re‑measure PVR weekly for the first four weeks.
- Continue quarterly checks as long as the drug is used.
- Discontinue immediately if PVR rises above 200 mL or the patient reports acute voiding difficulty.
Adding an α1‑blocker (e.g., tamsulosin) can reduce the retention risk by about 37 % in patients with BPH, according to a 2017 meta‑analysis. Transdermal oxybutynin patches also cut the risk by roughly 42 % versus oral tablets.
Alternative Therapies for Overactive Bladder
When the retention risk outweighs the benefit, clinicians turn to safer options:
- β3‑adrenergic agonists (mirabegron) - relax the detrusor without blocking M3.
- OnabotulinumtoxinA bladder injections - 0.5 % retention rate but require a specialist.
- Peripheral tibial nerve stimulation - non‑pharmacologic, minimal systemic effects.
- Behavioral therapies (bladder training, timed voiding) - useful as adjuncts.
These alternatives have become first‑line for men with prostate enlargement, while anticholinergics remain a mainstay for most women with OAB.
Practical Tips for Patients and Clinicians
Both sides can help keep retention at bay:
- Patient education: Teach the eight danger signs-straining, weak stream, feeling of incomplete emptying, frequent urgency, nocturia, dribbling, need to push, and inability to void for >12 hours.
- Medication review: Use the Anticholinergic Cognitive Burden (ACB) calculator to identify high‑scoring drugs.
- Dose titration: Start low, increase slowly, and pause if PVR climbs.
- Home bladder scanners: Telehealth programs (e.g., MyBladderMD) show >90 % adherence and cut emergency visits by 60 %.
- Documentation: Record baseline PVR, follow‑up values, and any catheterisations in the EMR.
By sticking to a clear monitoring protocol, the majority of patients avoid severe retention while still gaining symptom relief.
Quick Reference Table
| Parameter | Low‑risk threshold | High‑risk indication |
|---|---|---|
| Baseline PVR (mL) | ≤150 | >150 - consider alternative therapy |
| ACB score | 0‑2 | ≥3 - review medication list |
| Age | ≤65 | >65 with BPH - avoid non‑selective anticholinergics |
| Concurrent opioids | No | Yes - risk jumps to 12.7 % |
Frequently Asked Questions
What symptoms signal that an anticholinergic is causing urinary retention?
Common clues include a weak or intermittent stream, a feeling of incomplete emptying, sudden urgency followed by an inability to void, and the need to strain or use a catheter. If any of the eight danger signs appear, contact a healthcare provider right away.
Can I safely use an oral anticholinergic if I have mild BPH?
Mild BPH isn’t an automatic ban, but the AUA recommends a baseline PVR and a low starting dose. If the PVR stays below 150 mL and no symptoms emerge, the drug may be continued with close monitoring.
Are transdermal patches safer than oral tablets?
Yes. Clinical data show a 42 % lower incidence of retention with the oxybutynin patch because the steady‑state serum level avoids the high peaks that trigger detrusor inhibition.
What non‑drug options exist for overactive bladder?
Beta‑3 agonists (mirabegron), onabotulinumtoxinA injections, peripheral tibial nerve stimulation, and behavioural bladder training are all evidence‑based alternatives with far lower retention risk.
How does the Anticholinergic Cognitive Burden (ACB) scale help?
The ACB assigns a score (1‑3) to each medication based on its anticholinergic activity. A total score of 3 or higher flags patients who are at significantly higher risk for both cognitive decline and urinary retention, prompting a medication review.
Understanding the link between anticholinergic drugs and urinary retention lets clinicians balance symptom control with safety. By measuring PVR, using selective agents, and keeping an eye on the anticholinergic burden, most patients can enjoy relief without ending up on the catheter.
Brady Johnson
October 26, 2025 AT 11:30Wow, the sheer avalanche of data on anticholinergic burden feels like a slap in the face for anyone who thought prescribing was just a habit. The article drags us through receptor subtypes like a bad sequel, yet somehow still manages to drop the ball on patient‑centred nuance. It's almost heroic how the author tries to list every drug, but the execution reads like a spreadsheet on a bad Tuesday. If you asked me for a quick takeaway I'd say "watch those ACB scores like a hawk".
Jay Campbell
October 27, 2025 AT 15:16Appreciate the thorough overview. The baseline PVR recommendation is especially useful for primary care settings.
Laura Hibbard
October 28, 2025 AT 19:03Sure, because everyone has a PVR machine at home and checks it weekly, right?
Tim Waghorn
October 29, 2025 AT 22:50While the tone may appear casual, the clinical implications of a post‑void residual exceeding 200 mL are non‑trivial and merit prompt intervention per AUA guidelines. Moreover, the interaction between anticholinergic load and α1‑blockade should be quantified in future studies.
Lori Brown
October 31, 2025 AT 08:10Great summary! 👍 Keeping patients educated on those eight danger signs can really cut down emergency visits. 😊
Rhea Lesandra
November 1, 2025 AT 06:23Exactly-patient education is the cornerstone. A quick reminder: when you discuss the signs, use plain language and repeat the list twice to reinforce retention. Also, consider providing a printed handout; many patients forget verbal instructions after the appointment.
Kasey Marshall
November 2, 2025 AT 10:10Baseline PVR is a must start low and titrate up slowly
Dave Sykes
November 3, 2025 AT 11:10Spot on-dose titration saves a lot of hassle
Erin Leach
November 4, 2025 AT 17:43That really hits home. I've seen seniors get scared when they notice a weak stream and it's good to know there are clear steps to follow.
Erik Redli
November 6, 2025 AT 05:50Honestly the whole focus on anticholinergics is overblown; most patients tolerate them fine and the retention numbers are inflated by reporting bias.
Paul Luxford
November 7, 2025 AT 20:43I prefer a more conservative approach when prescribing to older men.
Nic Floyd
November 9, 2025 AT 14:23When we parse the pharmacodynamic landscape of muscarinic antagonists we encounter a spectrum of receptor affinity that directly informs urinary dynamics 🚀 The differential M3 selectivity of solifenacin versus the balanced profile of oxybutynin translates into a variance in detrusor suppression that clinicians must map onto patient‑specific risk matrices 📊 The anticholinergic cognitive burden score serves as a surrogate marker not only for neurocognitive outcomes but also for urodynamic perturbations especially in polypharmacy contexts 🌐 Empirical evidence from the JAGS 2017 cohort underscores a 70% elevation in retention odds at an ACB threshold of three or greater which dovetails with the mechanistic rationale of muscarinic blockade 🧪 Real‑world data further reveal that co‑administration of opioids amplifies urinary retention risk to double digits necessitating proactive bladder monitoring protocols 📈 The integration of home‑based bladder scanners into telehealth workflows has demonstrated adherence exceeding ninety percent while truncating acute catheterisation events by sixty percent an outcome that aligns with value‑based care objectives 💡 From a formulary perspective the migration towards β3‑adrenergic agonists such as mirabegron mitigates muscarinic interference while preserving detrusor relaxation albeit at a modest cost premium 💰 Clinical pathways now recommend a stepwise algorithm starting with behavioural interventions, progressing to low‑dose anticholinergics with stringent PVR checkpoints before escalating to neuromodulation techniques 🛤️ Moreover the pharmacokinetic profile of transdermal oxybutynin patches circumvents first‑pass metabolism, attenuating peak plasma concentrations that are implicated in acute retention episodes 🩹 In practice, the decision matrix should incorporate patient age, baseline PVR, comorbid BPH status and concomitant CNS depressants to stratify risk and individualize therapy 🧭 Future research ought to focus on longitudinal registries capturing real‑time PVR trends correlated with ACB fluctuations to refine predictive analytics 📚 Ultimately the goal is to harmonize symptomatic relief of overactive bladder with preservation of voiding efficiency thereby enhancing quality of life metrics 🎉 In addition, the role of genetics in metabolizing anticholinergics is emerging as a factor that could personalize dosing regimens. Standardized reporting of post‑void residual values across institutions would facilitate comparative effectiveness research. Finally, interdisciplinary collaboration between urologists, pharmacists and primary care physicians remains pivotal to mitigate adverse urinary outcomes.