Sorafenib Side Effect Management Tool
Manage Your Side Effects
Select your side effect and severity level to get evidence-based management recommendations.
When doctors prescribe Sorafenib is a multikinase inhibitor used mainly for advanced hepatocellular carcinoma (HCC) and renal cell carcinoma (RCC), patients often hear a lot of rumors. Some of those rumors are harmless, but others can lead to skipped doses, unnecessary worries, or even dangerous drug interactions. Below we clear up the most frequent misconceptions so you can stay on track with your sorafenib therapy and get the best possible outcome.
What fuels the myths?
Misunderstandings typically arise from three sources:
- Complex biology: Sorafenib blocks several pathways (VEGFR, PDGFR, RAF kinases) that most patients can’t visualize.
- Side‑effect overlap: Symptoms like hand‑foot skin reaction look similar to reactions from other drugs, leading to mix‑ups.
- Information gaps: Oncology clinics often hand out dense pamphlets that patients skim, missing key details.
Let’s tackle each myth head‑on.
Myth 1 - "Sorafenib is a cure, not a control"
Reality: Sorafenib is a **disease‑control** agent. Clinical trials (e.g., SHARP study, 2008) showed a median overall survival extension of about 2.8 months for HCC patients, not a cure. The drug slows tumor growth by inhibiting angiogenesis via VEGFR‑2 and RAF/MEK pathways.
Why it matters: Expecting a cure can cause disappointment and early discontinuation. Knowing that the goal is to **prolong survival and improve quality of life** helps patients stick with the regimen.
Myth 2 - "If I miss a dose, the treatment stops working"
Reality: Missing an occasional dose does not instantly erase the drug’s benefit, but consistent non‑adherence reduces progression‑free survival. Pharmacokinetic data indicate a half‑life of ~25-48 hours; the drug accumulates in tissue, so a single missed pill isn’t catastrophic.
Best practice: If you forget a dose, take it as soon as you remember-unless it’s within 8‑12 hours of the next scheduled dose, then skip the missed one and continue as usual. Document missed doses in a medication diary to discuss with your oncology nurse.
Myth 3 - "Sorafenib can be taken with any food or drink"
Reality: Sorafenib should be taken on an empty stomach-at least one hour before or two hours after meals. Food, especially high‑fat meals, can lower oral bioavailability by up to 30 %.
Practical tip: Set a reminder to take the capsule with a glass of water, then wait before breakfast or after lunch. This small habit ensures steady drug levels.
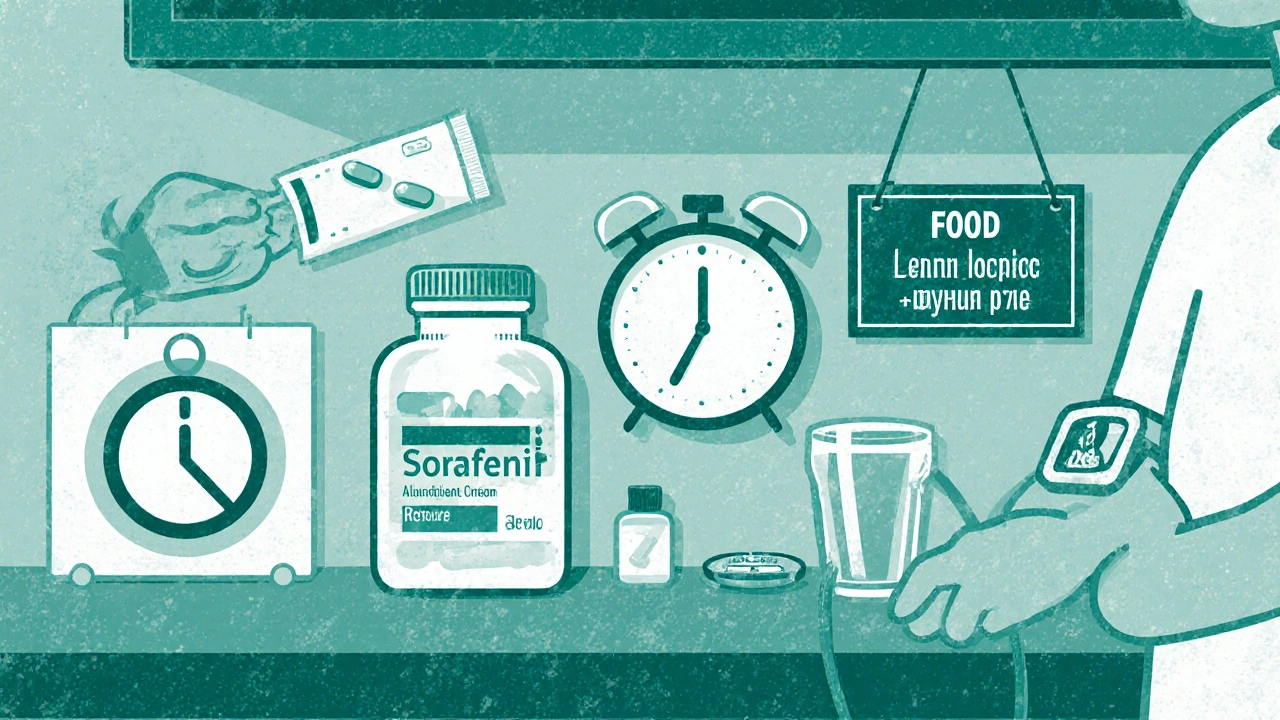
Myth 4 - "All side effects are severe and irreversible"
Reality: While side effects are common, most are manageable and reversible with dose adjustments or supportive care. The most reported adverse events include:
- Hand‑foot skin reaction - often improves with emollients and dose reduction.
- Diarrhea - can be mitigated with loperamide and dietary tweaks.
- Hypertension - monitor blood pressure weekly; antihypertensives work well.
Important nuance: Severe grade 3-4 toxicities (< 5 % of patients) may require temporary interruption or permanent discontinuation, but early reporting prevents escalation.
Myth 5 - "Sorafenib works the same for every cancer"
Reality: Sorafenib’s efficacy varies by tumor type and molecular profile. In HCC, the drug targets VEGFR‑2, PDGFR‑β, and RAF kinases, leading to modest survival benefit. In RCC, it chiefly inhibits VEGFR‑2 and improves progression‑free survival, but not overall survival as dramatically. Ongoing research suggests that patients with specific BRAF‑mutated tumors may respond better, yet this isn’t a universal rule.
Takeaway: Your oncologist decides on sorafenib based on imaging, lab values, and sometimes genetic testing. The drug isn’t a one‑size‑fits‑all solution.
Myth 6 - "I don’t need regular lab tests while on sorafenib"
Reality: Routine monitoring is critical. Sorafenib can affect liver enzymes, thyroid function, and electrolytes. Standard protocol includes:
- Baseline liver function tests (ALT, AST, bilirubin) and then every 2‑4 weeks.
- Blood pressure checks weekly for the first two months.
- Thyroid‑stimulating hormone (TSH) every 3 months.
Skipping labs may hide rising bilirubin or hypertension, which can become dangerous if left untreated.
Myth 7 - "Sorafenib has no major drug interactions"
Reality: Sorafenib is metabolized by CYP3A4 and is a substrate for P‑gp transporters. Strong CYP3A4 inducers (e.g., rifampin, carbamazepine) can lower its plasma concentration, while strong inhibitors (e.g., ketoconazole, grapefruit juice) can raise levels and increase toxicity.
Key rule: Always inform your healthcare team about over‑the‑counter meds, herbal supplements, and dietary changes. A quick screen for CYP3A4‑affecting agents can prevent unexpected side effects.
Quick Myth‑Fact Table
| Myth | Fact |
|---|---|
| Sorafenib cures cancer | It prolongs survival but does not eradicate disease. |
| Missing one dose stops treatment | Occasional missed doses are tolerable; consistency matters. |
| Can be taken with meals | Best on an empty stomach for optimal absorption. |
| All side effects are permanent | Most are reversible with dose tweaks and supportive care. |
| Works identically for every tumor | Efficacy depends on cancer type and molecular profile. |
| No lab monitoring needed | Regular liver, blood pressure, and thyroid tests are essential. |
| No drug interactions | Interacts with CYP3A4 modulators and P‑gp substrates. |
Patient Checklist for Safe Sorafenib Use
- Take 400 mg twice daily on an empty stomach.
- Mark your calendar for lab draws: liver panel every 2 weeks, BP weekly, TSH quarterly.
- Keep a side‑effect log; report grade 2 or higher skin changes immediately.
- Review all concurrent meds with your pharmacist-especially antibiotics, antifungals, and St. John’s wort.
- Stay hydrated; mild diarrhea is common but severe dehydration can raise drug levels.
When to Call Your Healthcare Team
Even with proper education, red‑flag symptoms pop up. Call the oncology clinic if you notice:
- Sudden increase in blood pressure (> 160/100 mmHg) despite medication.
- Severe hand‑foot skin reaction preventing daily activities.
- Yellowing of skin or eyes (possible liver toxicity).
- Unexplained fever, chills, or severe abdominal pain.
Quick intervention can keep you on therapy safely.
Frequently Asked Questions
Can I take sorafenib if I have mild liver impairment?
Mild impairment (Child‑Pugh A) is usually acceptable, but dose adjustments may be needed. Severe impairment (Child‑Pugh B/C) typically contraindicates use because the drug’s clearance drops, raising toxicity risk.
How long should I stay on sorafenib after tumor progression?
Continuation is considered if the drug still controls other disease sites or symptoms. Many oncologists switch to second‑line agents but may keep sorafenib at a reduced dose if it provides clinical benefit.
Is it safe to combine sorafenib with immunotherapy?
Early trials (e.g., KEYNOTE‑524) show promising activity, but the combination raises the risk of liver toxicity. Such regimens should only be used within a clinical trial or under close specialist supervision.
What should I do if I develop a grade 3 hand‑foot skin reaction?
Stop sorafenib immediately, apply thick moisturizing creams, and contact your oncology nurse. Dose reduction or temporary discontinuation is typical until the reaction drops to grade 1‑2, then restart at a lower dose.
Do I need to avoid grapefruit while on sorafenib?
Yes. Grapefruit juice inhibits CYP3A4, which can raise sorafenib plasma levels and increase side effects. Stick to non‑citrus fruits like berries or apples.
By separating fact from fiction, you can navigate sorafenib therapy with confidence, keep side effects manageable, and stay on track for the longest possible benefit.
Michael Vandiver
October 22, 2025 AT 19:10Thanks for breaking down the sorafenib myths! 😊 It's so easy to get confused with all the jargon but your table makes it crystal clear 👍 I love the tip about taking the pill on an empty stomach – set a reminder on my phone now 🚀 Keep the great info coming!
Harini Prakash
October 22, 2025 AT 20:33Really helpful guide, especially the checklist at the end :) The part about monitoring blood pressure weekly is a good reminder for many of us. Keeping a side‑effect log can make a huge difference in getting timely help.
Rachael Turner
October 22, 2025 AT 21:56Reading through the myths feels like peeling back layers of a complex story. Each clarification not only informs but also empowers patients to participate actively in their treatment journey. Understanding that sorafenib is a disease‑control agent rather than a cure reshapes expectations and reduces unnecessary disappointment.
Suryadevan Vasu
October 22, 2025 AT 23:20Take sorafenib on an empty stomach; avoid high‑fat meals to maintain absorption.
Vin Alls
October 23, 2025 AT 00:43Sorafenib is a fascinating molecule that walks the tightrope between efficacy and toxicity.
Its ability to shut down multiple kinases makes it a multitool in the oncologist's arsenal.
However, this very breadth of action is the source of many misconceptions among patients.
When you hear that it 'cures' cancer, the reality is that it merely extends survival by a modest margin, buying precious time.
Pharmacokinetically, the drug lingers in tissues for days, which is why missing an occasional dose does not immediately erase its benefits.
Nonetheless, consistency matters; the cumulative exposure over weeks determines the true therapeutic impact.
Food is a sneaky adversary – a greasy breakfast can shave off up to thirty percent of the drug's bioavailability.
That is why the prescription label insists on an empty‑stomach regimen, a small habit with big payoff.
Side effects such as hand‑foot skin reaction or hypertension are not destiny; they are manageable with dose tweaks and supportive care.
A simple moisturizer and a blood‑pressure cuff at home can prevent the minor from becoming major.
Laboratory monitoring is non‑negotiable, especially liver enzymes, which can flare without warning.
Regular checks every two to four weeks act as an early warning system, allowing dose adjustments before toxicity spirals.
Drug interactions are a hidden minefield; CYP3A4 inducers like rifampin can dilute sorafenib's potency, while grapefruit juice can amplify its toxicity.
Always hand over a complete medication list to your oncology team, including herbal supplements, to stay clear of dangerous synergisms.
In the end, sorafenib is not a silver bullet, but when wielded correctly, it is a valuable ally in the fight against liver and kidney cancers.
Tiffany Davis
October 23, 2025 AT 02:06I agree, the weekly blood pressure checks are essential and often overlooked.
Don Goodman-Wilson
October 23, 2025 AT 03:30Great, another miracle cure that only adds a few months.
Bret Toadabush
October 23, 2025 AT 04:53They dont tell u that big pharma pushes sorafenib just to keep the cash flow going, while hiding better natural options.
Iris Joy
October 23, 2025 AT 06:16While it's natural to be skeptical, clinical trials have demonstrated sorafenib's benefit, and any alternative should be weighed against solid evidence. Keeping an open mind and discussing options with your doctor is the safest path.
Jai Reed
October 23, 2025 AT 07:40For patients starting sorafenib, creating a medication diary can be invaluable; record dose times, food intake, side effects, and any over‑the‑counter products. This documentation not only helps you stay organized but also provides your care team with precise data to tailor therapy safely.